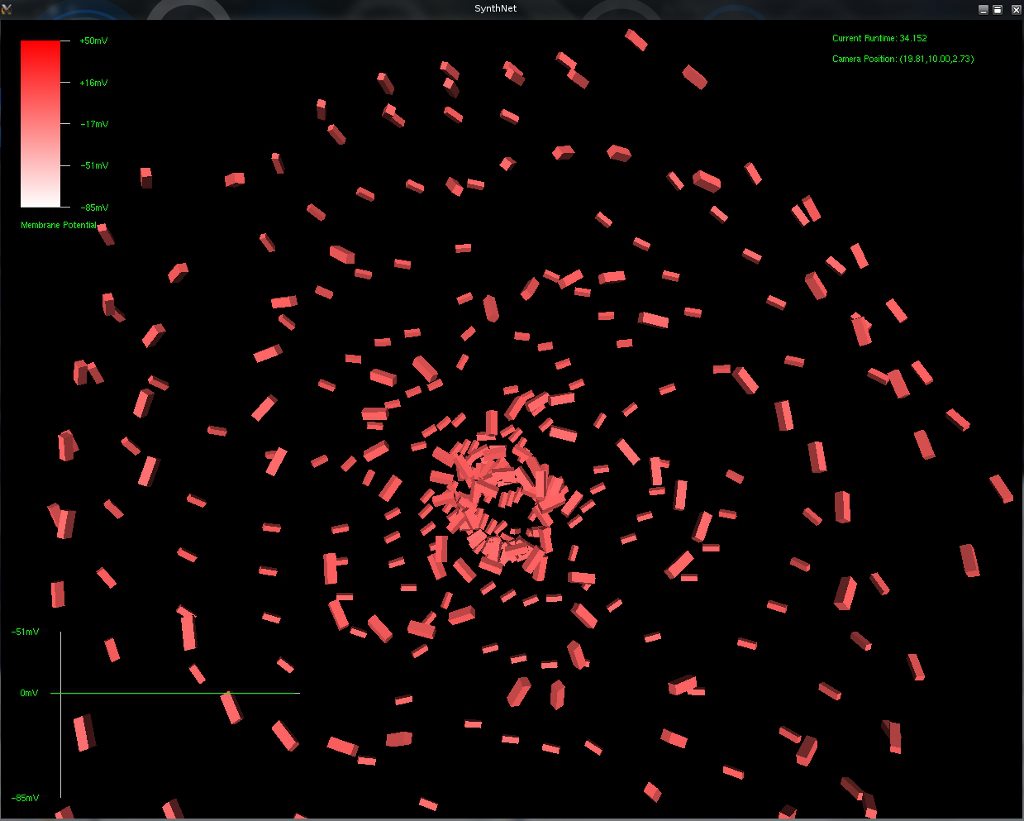
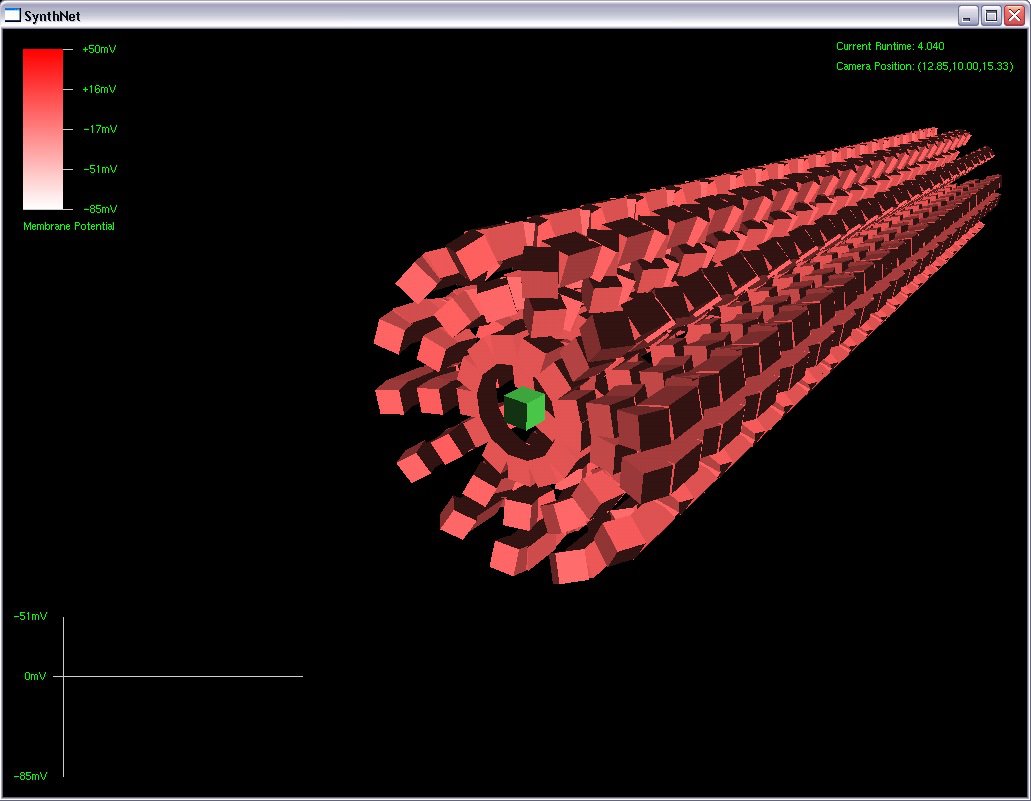
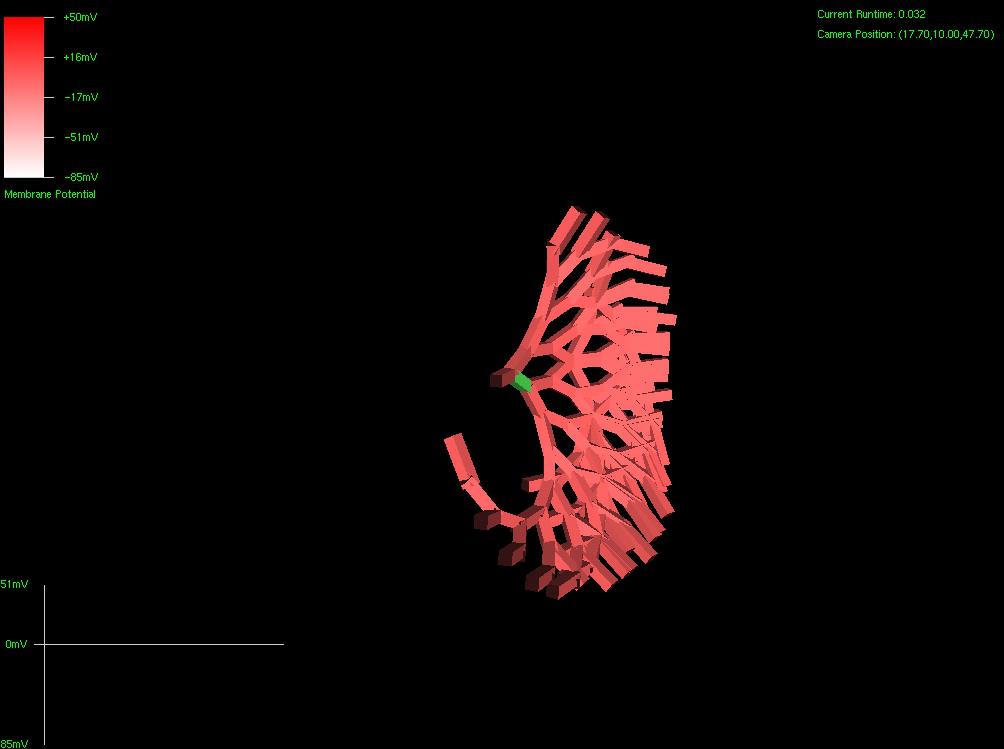
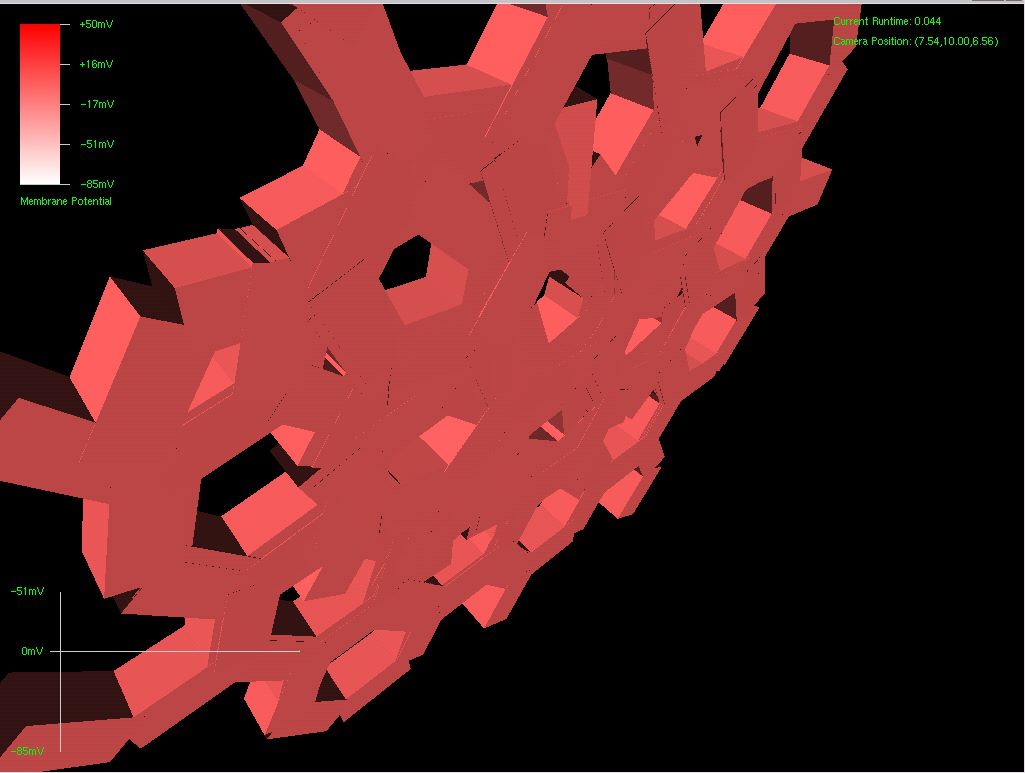
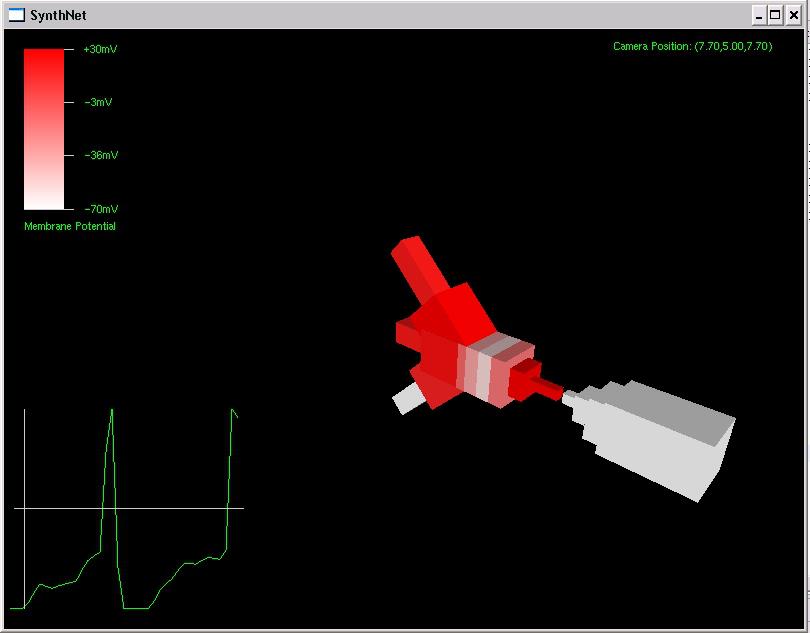
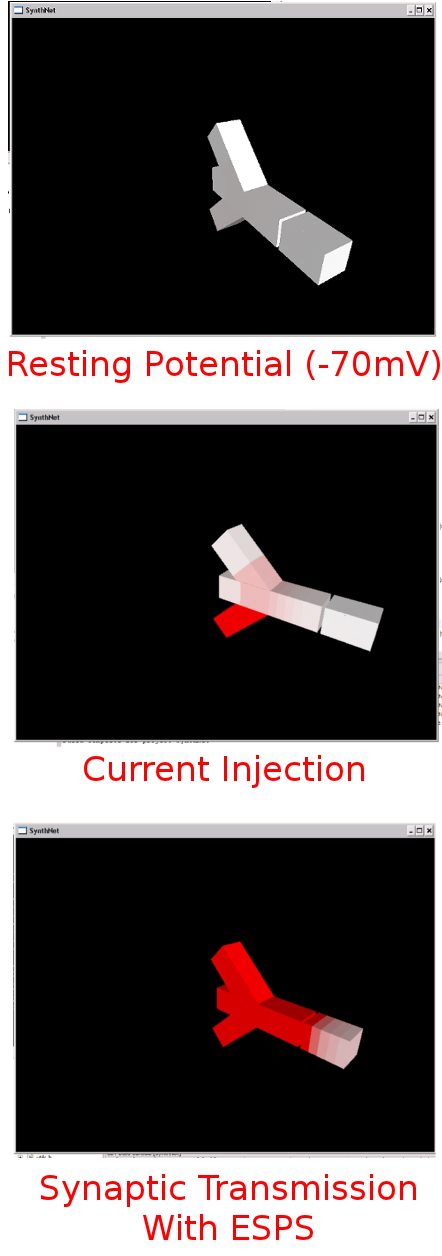
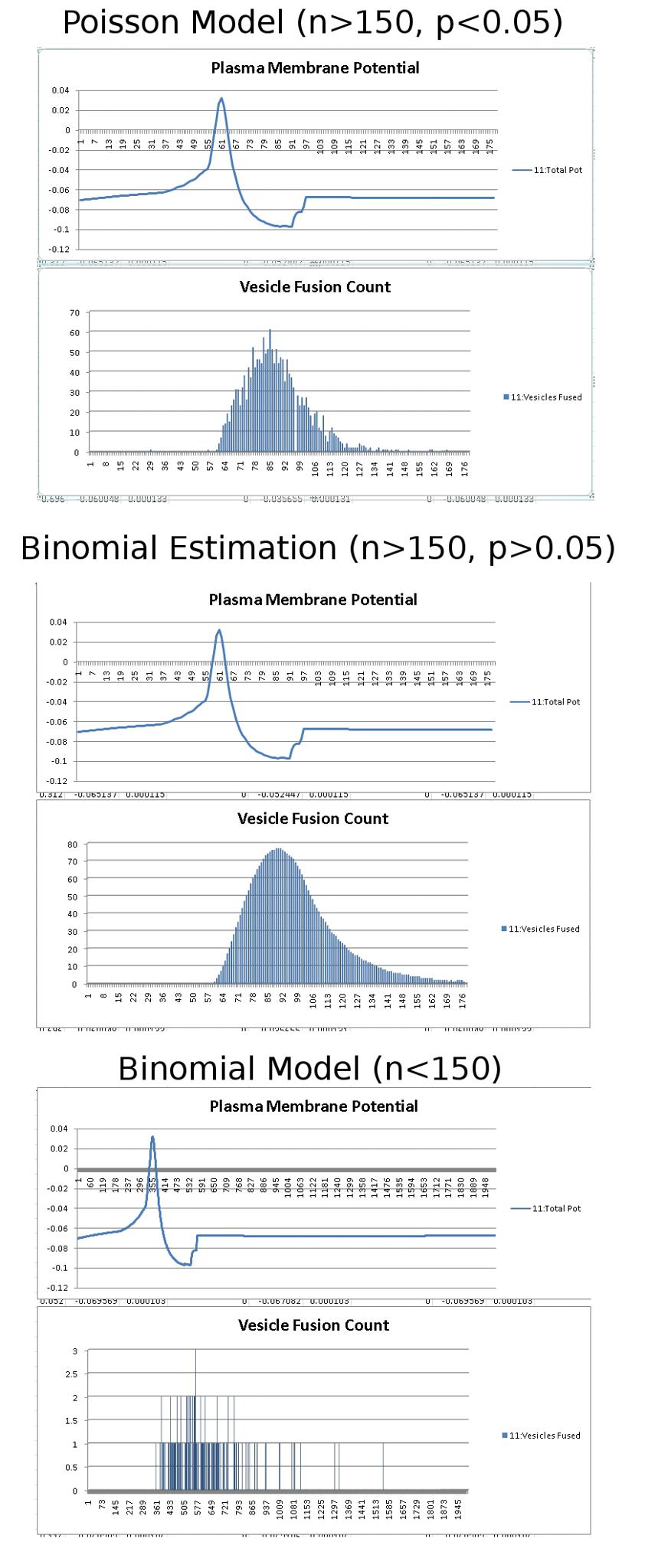
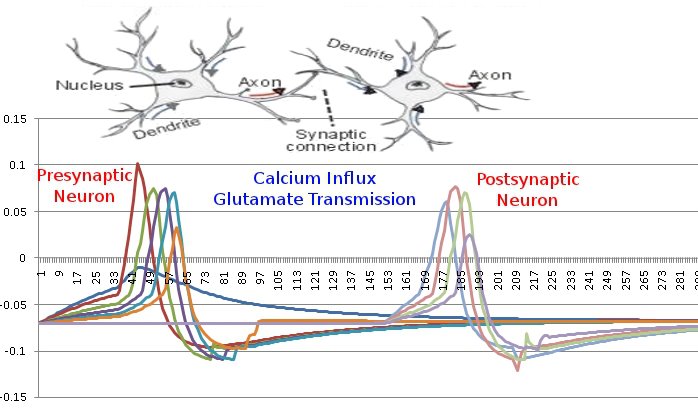
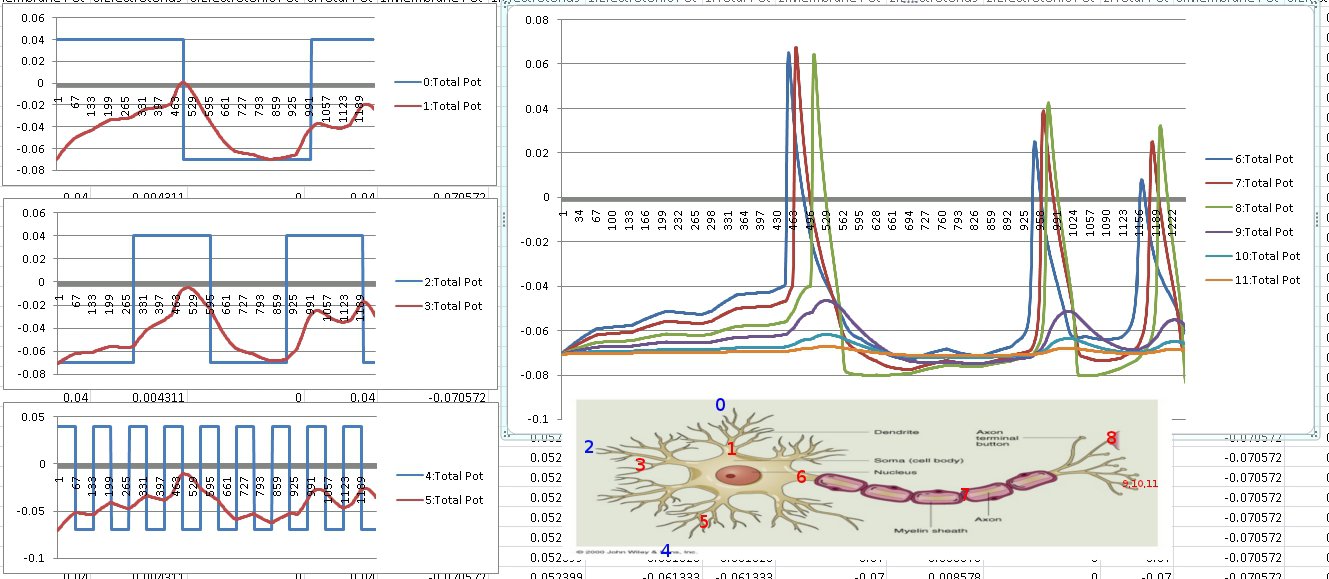
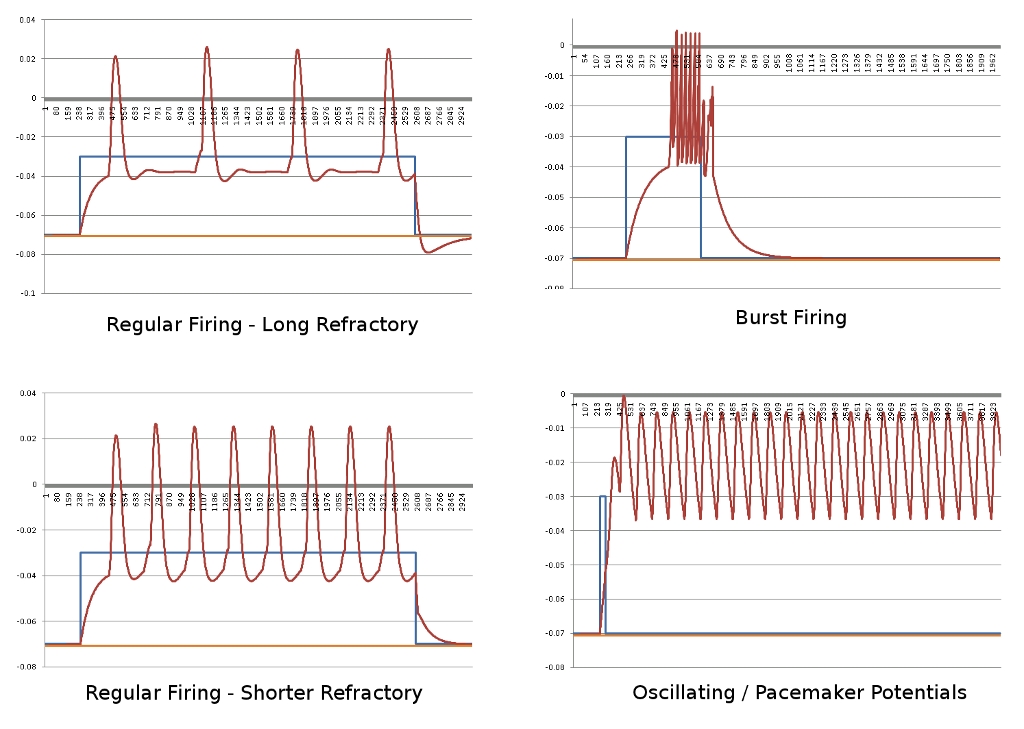
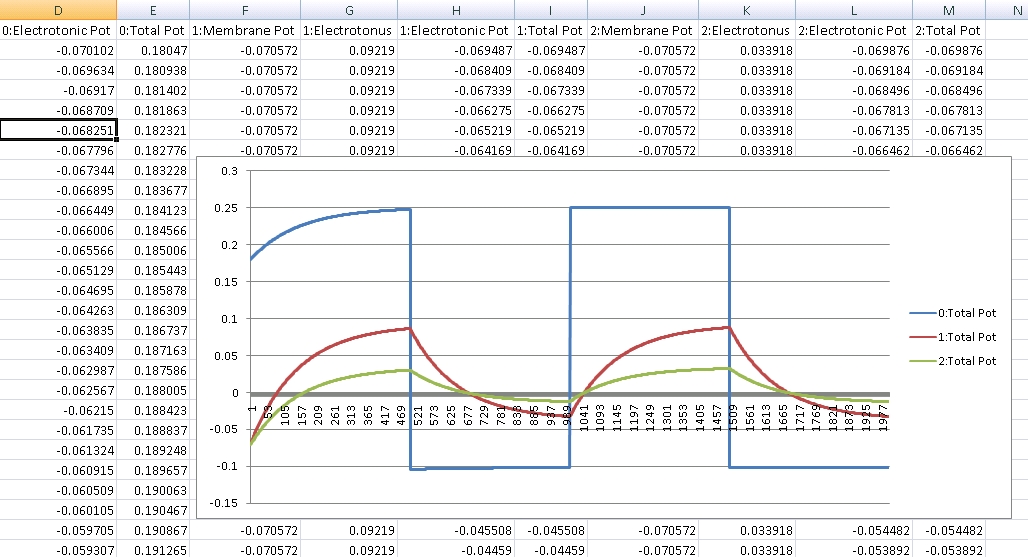
Since the genetic engine is pretty much finished up now, I’ve started on some loose ends of things I want to implement in SynthNet. This is a stress test for the (start of the) new multithreading capabilities – using a simple strand of DNA to direct a base stem cell to continually go through mitosis and differentiate. These daughter cells then follow a genetically programmed spiraling migration path. You can see patterns start to emerge amongst the thousands of cells.
Some parts of the processing engine are crashing out right now, so it’s apparent I’m having some kind of issues arising from sharing information between threads – I’m going to shelve the functionality for now and then complete it up after the end of phase 1 – there may be quite a bit involved.